Key Market Insights
- Presently, more than 70 companies claim to have the required expertise to offer various services for the synthesis and manufacturing of mRNAs across different scales of operations, worldwide
- In pursuit of building a competitive edge, service providers are actively upgrading their existing capabilities to enhance their respective portfolios and comply with the evolving industry benchmarks
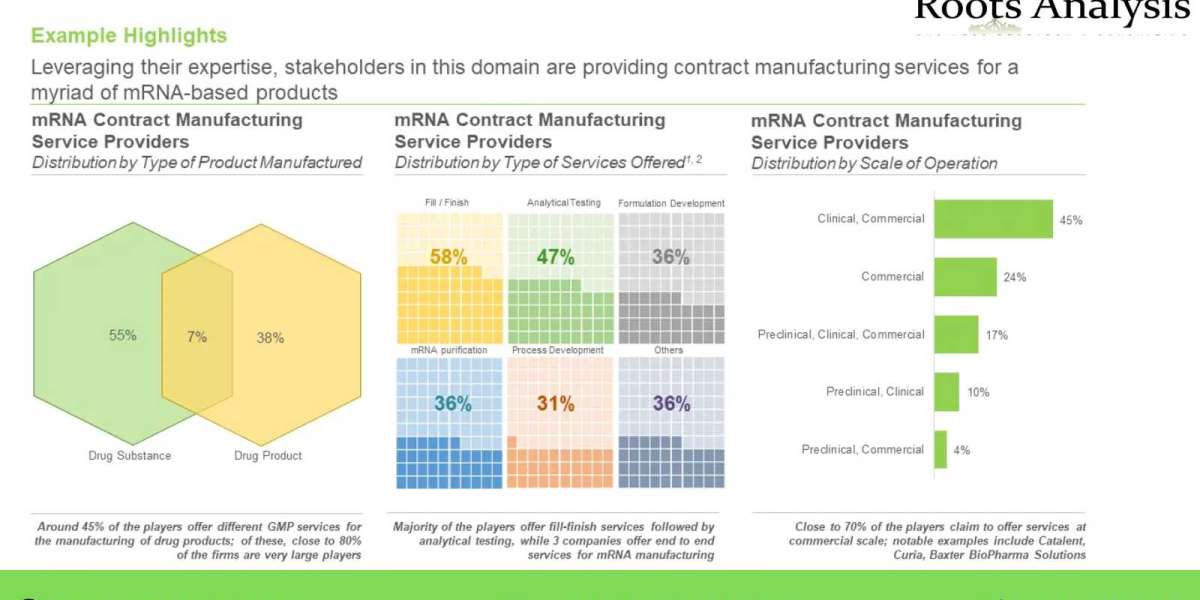
- Leveraging their expertise, stakeholders in this domain are providing contract manufacturing services for a myriad of mRNA-based products
- Presently, more than 95 kits are available in the market for the synthesis of research grade mRNAs used for various applications with yield range up to 50,000 µgs
- Close to 50% of mRNA synthesis kits contain all the components, including enzyme mix, buffers and other reagents, required for the synthesis of varying quantity of modified mRNAs at affordable prices
- mRNA synthesis kit providers are steadily expanding their capabilities in order to enhance their existing capabilities and augment their respective kit portfolios
- A case study of considerable increase in the number of clinical trials registered for mRNA-based therapeutics / vaccines shows growing demand for such therapeutic / preventive interventions
- The growing interest is evident from the rise in partnership activity; in fact, the maximum number of collaborations related to mRNA-based drug manufacturing were inked in 2020 and 2021
- Close to 35 mRNA-based therapeutic / vaccine developers across the world are anticipated to forge strategic alliances with mRNA synthesis and mRNA manufacturing service providers, to further augment their drug portfolio
- In order to tap the lucrative opportunity in this rapidly growing market, big pharma players have undertaken several initiatives, including strengthening product portfolio, agreements, and investments
- The market’s evolution is likely to be driven by the need for novel mRNA therapeutics; we expect the future opportunity to be well distributed across various types of product, therapeutic areas, and key geographical regions
- PREFACE
1.1. Scope of the Report
1.2. Market Segmentations
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. An Overview of mRNA
3.2. Structure of mRNA
3.3. Evolution of mRNA Vaccines
3.4. mRNA Manufacturing Process
3.4.1. Chemical Synthesis of mRNA
3.4.2. In vitro Synthesis of mRNA
3.5. Applications of Chemically / in vitro Synthesized mRNA
3.6. Challenges Associated with mRNA Synthesis
3.7. Commonly Outsourced Manufacturing Operations
3.8. Advantages of Outsourcing Manufacturing Operations
- MARKET LANDSCAPE: mRNA CUSTOM SYNTHESIS SERVICE PROVIDERS
4.1. mRNA Custom Synthesis Service Providers: Overall Market Landscape
4.1.1. Analysis by Year of Establishment
4.1.2. Analysis by Company Size
4.1.3. Analysis by Location of Headquarters
4.1.4. Analysis by Company Size and Location of Headquarters
4.1.5. Analysis by Type of Service(s) Offered
4.1.6. Analysis by Input for Synthesis
4.1.7. Analysis by Structural Modification
4.1.8. Analysis by Type of Purification Method(s)
4.1.9. Analysis by Application Area(s)
4.1.10. Analysis by Scale of Operation
4.1.11. mRNA Custom Synthesis Service Providers: Information on Length (Base Pair) of mRNA Manufactured
4.1.12. mRNA Custom Synthesis Service Providers: Information on mRNA Synthesis / Manufacturing Capacity
- mRNA CUSTOM SYNTHESIS SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
5.1. Key Parameters and Scores
5.2. Methodology
5.3. Competitiveness Analysis: Small Companies
5.4. Competitiveness Analysis: Mid-Sized Companies
5.5. Competitiveness Analysis: Large Companies
- MARKET LANDSCAPE: mRNA CONTRACT MANUFACTURING SERVICE PROVIDERS
6.1. mRNA Contract Manufacturing Service Providers: Overall Market Landscape
6.1.1. Analysis by Year of Establishment
6.1.2. Analysis by Company Size
6.1.3. Analysis by Location of Headquarters
6.1.4. Analysis by Company Size and Location of Headquarters
6.1.5. Analysis by Location of Manufacturing Facility
6.1.6. Analysis by Type of Product(s) Manufactured
6.1.7. Analysis by Type of Service(s) Offered
6.1.8. Analysis by Scale of Operation
- mRNA CONTRACT MANUFACTURING SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
7.1. Methodology
7.2. Company Competitiveness Analysis: mRNA Contract Manufacturing Service Providers based in North America
7.3. Company Competitiveness Analysis: mRNA Contract Manufacturing Service Providers based in Europe
7.4. Company Competitiveness Analysis: mRNA Contract Manufacturing Service Providers based in Asia-Pacific
- MARKET LANDSCAPE: mRNA SYNTHESIS KIT PROVIDERS
8.1. mRNA Synthesis Kits: Overall Market Landscape
8.1.1. Analysis by Kit Components
8.1.2. Analysis by Type of Enzyme
8.1.3. Analysis by Type of Enzyme Mix Used
8.1.4. Analysis by mRNA Component Modified
8.1.5. Analysis by Yield per Reaction
8.1.6. Analysis by Number of Reactions
8.1.7. Analysis by Reaction Run-Time
8.1.8. Analysis by Price of Kits
8.1.9. Most Active Players: Analysis by Number of mRNA Synthesis Kits Offered
8.2. mRNA Synthesis Kits: Developer Landscape
8.2.1. Analysis by Year of Establishment
8.2.2. Analysis by Company Size
8.2.3. Analysis by Location of Headquarters
- mRNA SYNTHESIS KITS: PRODUCT COMPETITIVENESS ANALYSIS
9.1. Methodology
9.2. Product Competitiveness Analysis: mRNA Synthesis Kits
- COMPANY PROFILES: mRNA SYNTHESIS AND MANUFACTURING SERVICE PROVIDERS
10.1. Aldevron
10.2. Biomay
10.3. bioSYNTHESIS
10.4. eTheRNA
10.5. Eurogentec
10.6. TriLink BioTechnologies
- COMPANY PROFILES: mRNA SYNTHESIS KIT PROVIDERS
11.1. APExBIO
11.2. CELLSCRIPT
11.3. Jena Biosciences
11.4. New England Biolabs
11.5. Thermo Fisher Scientific
- CLINICAL TRIAL ANALYSIS
12.1. Methodology
12.2. mRNA-based Therapeutics / Vaccines: Clinical Trial Analysis
12.2.1. Analysis by Trial Registration Year
12.2.2 Analysis by Trial Status
12.2.3. Analysis by Trial Registration Year and Patients Enrolled
12.2.4. Analysis by Trial Registration Year and Trial Status
12.2.5. Analysis by Trial Phase
12.2.6. Analysis by Target Patient Population
12.2.7. Analysis by Therapeutic Area
12.2.8. Analysis by Type of Sponsor / Collaborator
12.2.9. Most Active Players: Analysis by Number of Trials
12.2.10. Analysis by Region
- PARTNERSHIP AND COLLABORATIONS
13.1. Partnership Models
13.2. mRNA Synthesis and Manufacturing: Partnerships and Collaborations
13.1.1. Analysis by Year of Partnership
13.1.2. Analysis by Type of Partnership
13.1.3. Analysis by Type of Product
13.1.4. Analysis by Type of Partner
13.1.5. Most Active Players: Analysis by Number of Partnerships
13.1.6. Analysis by Local and International Agreements
13.1.7. Analysis by Intercontinental and Intracontinental Agreements
13.1.8. Analysis by Region
- LIKELY PARTNERS ANALYSIS
14.1. Methodology
14.2. Likely Partners based in North America
14.3. Likely Partners based in Europe
14.4. Likely Partners based in Asia Pacific and Rest of the World
- BIG PHARMA INITIATIVES
15.1. Methodology
15.2. Big Pharma Players: List of mRNA-based Therapeutics / Vaccines Focused Initiatives
15.3. Competitive Benchmarking of Big Pharma Players
15.3.1. Harvey Ball Analysis
15.3.2. Spider Web Analysis
- MARKET FORECAST
16.1. Chapter Overview
16.2. Key Assumptions and Forecast Methodology
16.3. Global mRNA Synthesis and Manufacturing Market, 2022-2035
16.3.1. mRNA Synthesis and Manufacturing Market, 2022-2035: Distribution by Type of Product
16.3.1.1. mRNA Synthesis and Manufacturing Market for Drug Substances (APIs), 2022-2035
16.3.1.2. mRNA Synthesis and Manufacturing Market for Drug Products (FDFs), 2022-2035
16.3.2. mRNA Synthesis and Manufacturing Market, 2022-2035: Distribution by Application Area,
16.3.2.1. mRNA Synthesis and Manufacturing Market for mRNA-based Vaccines, 2022-2035
16.3.2.2. mRNA Synthesis and Manufacturing Market for mRNA-based Therapeutics, 2022-2035
16.3.3. mRNA Synthesis and Manufacturing Market: Distribution by Therapeutic Area, 2022, 2025 and 2035
16.3.3.1. mRNA Synthesis and Manufacturing Market for Infectious Diseases, 2022-2035
16.3.3.1. mRNA Synthesis and Manufacturing Market for Oncological Disorders, 2022-2035
16.3.3.1. mRNA Synthesis and Manufacturing Market for Other Diseases, 2022-2035
16.3.4. mRNA Synthesis and Manufacturing Market: Distribution by Geography, 2025 and 2035 (USD Billion)
16.3.4.1. mRNA Synthesis and Manufacturing Market in North America, 2022-2035
16.3.4.2. mRNA Synthesis and Manufacturing Market in Europe, 2022-2035
16.3.4.3. mRNA Synthesis and Manufacturing Market in Asia Pacific, 2022-2035
16.3.4.4. mRNA Synthesis and Manufacturing Market in Latin America, 2022-2035
16.3.4.5. mRNA Synthesis and Manufacturing Market in MENA, 2022-2035
16.3.4.6. mRNA Synthesis and Manufacturing Market in Rest of the World, 2022-2035
- EXECUTIVE INSIGHTS
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/mrna-synthesis-and-manufacturing-market.html
You may also be interested in the following titles:
You may also like to learn what our experts are sharing in Roots educational series:
Antiviral Drugs Development: Are we Prepared for the Next Viral Pandemic? |
Exploring How Artificial Intelligence Is Transforming Digital Pathology |
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415